
Precise targeting for TMS

Precise targeting for TMS treatment
ANT Neuro's FDA cleared visor2* TMS Neuronavigation system allows clinicians to deliver accurate, consistent, and personalized TMS sessions
Discover the power of visor2™ today
ANT Neuro's FDA cleared visor2 TMS Neuronavigation system allows to deliver more accurate, consistent, and personalized navigated TMS therapy
Best in class TMS Neuronavigation
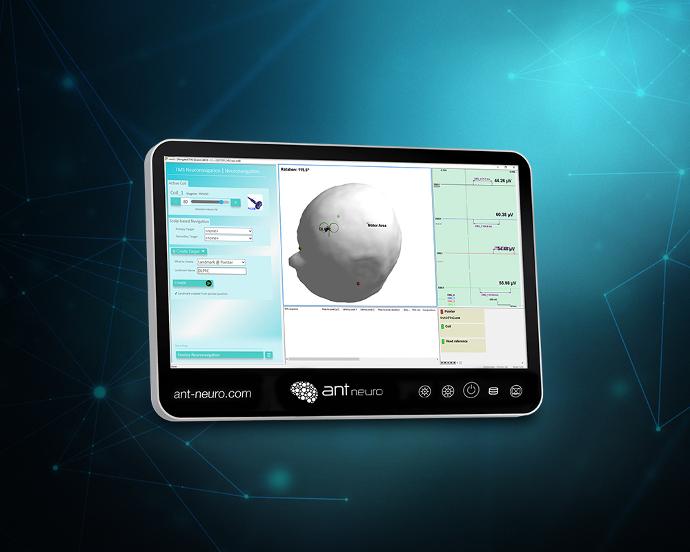

Streamlined motor threshold determination, and precise, reproducible targeting of TMS pulses
targeting of TMS pulses.
The optional motor threshold measurement workflow in visor2™ facilitates fast and accurate measurement of a patient's motor threshold.
visor2's™ target helper tool guides the clinician to precisely place the TMS coil over user-defined treatment targets.
targets.
measurement workflow in visor2 facilitates fast and accurate measurement of a patient's motor threshold.
Personalized navigation with or without individual MRI or fMRI
visor2™* enables TMS providers to use an individual patient's brain image (MRI) or functional evaluation (fMRI) to define treatment targets and navigate the TMS coil based on the patient's unique neuroanatomy.
When a patient's MRI is unavailable a simple scalp-based navigation workflow using the 5.5cm rule guides placement of the TMS coil.

Compatible with most FDA 510(k) approved TMS systems
visor2™ seamlessly integrates with a wide range of TMS systems, including Magstim (Horizon), MagVenture, neurocare (Apollo), and Neuronetics (Neurostar). This open approach ensures that the visor2™ system will be compatible with your current TMS device without locking you in to a particular system.
Reusable tracking tools allow integration into your existing TMS setups with no recurring costs.
Discover the power of visor2™ today
*visor2™ system is CE marked medical device (CE Class IIa) under MDR (EU) 2017/745, has FDA clearance under 510(k) in the USA, Medical Device License (MDL) issued by Health Canada and is registered in the ARTG in compliance with the Australian TG(MD)R. EEG-TMS and dual-coil navigation with visor2 is intended for research and educational use only.
Information intended for users in USA: Conditions that are FDA cleared for treatment with TMS are specific to each stimulation device. Please consult your stimulator manufacturer for more information. Manufactured by eemagine Medical Imaging Solutions GmbH, Berlin, Germany, ISO 13485 certified. ANT Neuro and eemagine are part of the neuromotion group. For more information about visor2 and the regulatory status in your country, contact us at [email protected].

