Dr. Robert Fleischmann, a neurologist at the University of Greifswald, researches disorders of consciousness and motor system plasticity after stroke. His work explores the use of dry EEG technology as a faster, more comfortable alternative to traditional gel EEG, particularly in clinical populations like stroke and delirium patients. Studies show dry EEG performs comparably in cognitive tasks and resting state connectivity, despite some signal-to-noise limitations. Its ease of use makes it suitable for multi-center trials, ecological settings, and populations sensitive to lengthy procedures, such as long COVID or ME/CFS patients. Dry EEG also holds promise for large-scale data collection and AI-driven neuroscience research.
Meet the Researcher

Dr. Robert Fleischmann is a senior physician working in the Neurology department of the University of Greifswald since 2019. He has had a prolific career and contributed to the department of neurology in Charité, Universitätsmedizin Berlin through his research for stroke, headache, and neurology between 2011–2017. Dr. Fleischmann has also held positions in the departments of Psychiatry and Neurology in the years between 2017–2019. His research focuses on disorders of consciousness such as delirium, investigating neurophysiological biomarkers of headache disorders and underpinnings of the physiology and plasticity of the motor system in post-stroke subjects.
Using Dry EEG in Clinical Contexts
Commercially available EEG systems use silver/silver chloride electrodes that require a conduction medium such as gel, paste or saline solution. The use of this increases application time or demands comprehensive preparation to optimize impedances and minimize measurement errors due to movement artifacts, gel bridges etc. Due to this, novel electrode technologies make dry or semi-dry contact electrodes a more convenient choice to work with, keeping the application time limited to a few minutes while ensuring subject comfort and compliance [1]. Recently, there have been many attempts to validate dry EEG against conventional gel EEG with positive outcomes that in turn motivate the EEG community to consider making the switch to high quality, multipin dry EEG technology. For example, dry EEG and wet EEG produce similar results in resting state EEG, especially at higher frequency alpha and beta bands.
Applicability of Dry EEG in Clinical Populations
The use of dry EEG also makes it possible to record from certain clinical subject populations that cannot cooperate with longer preparation times demanded by gel EEG. In Dr. Fleischmann’s studies we see dry EEG being applied to healthy subjects without any underlying psychiatric or neurological impairments, as well as subjects diagnosed with delirium. Delirium affects 20–30% of hospitalized older adults and is an acute disturbance in attention, awareness, and cognition caused due to the presence of other medical conditions. Management of delirium is imperative to avoid complications in the in-hospital period (e.g. falls, device displacement, pneumonia, thromboembolism) and associated risks of long-term cognitive dysfunction in an aging population. However, any understanding of the pathophysiology is limited due to fast, timely non-invasive neuroimaging procedures. Dry EEG makes it easier to investigate neurophysiological changes associated with delirium through quantitative EEG (qEEG) parameters such as spectral power distribution, peak frequency and functional connectivity measures such as phase lag index (PLI) which have been studied previously in delirious patients. Dry EEG, with its ease of use and adoption rate amongst subjects, makes it possible to also conduct multi-center trials to compare qEEG measures between delirious and non-delirious subject groups using standardized methodologies [2].
Due to this, novel electrode technologies make dry or semi-dry contact electrodes a more convenient choice to work with, keeping the application time limited to a few minutes while ensuring subject comfort and compliance.
Comparing Dry vs. Gel EEG Outcomes in Clinical Populations
Evidence across multiple validation and clinical studies conducted to compare gel EEG data with dry EEG helps us understand the impact that dry EEG could have when it comes to understanding neurophysiological underpinnings of a clinical condition. In this section, we refer to some published or soon-to-be published studies wherein we see promising results.
Comparison of Dry and Wet Encephalography for the Assessment of Cognitive Evoked Potentials and Sensor Level Connectivity
In this study, an auditory oddball paradigm and a mismatch negativity (MMN) paradigm were used to evaluate the performance of gel and dry EEG for a healthy subject group. Thirty-three healthy participants were recruited through public advertisements.
Experiment Procedure
The oddball paradigm was created using E-Prime and the standard stimulus was a 1,000 Hz tone, while deviant stimuli had frequencies of either 500 Hz or 1,500 Hz. Consecutive stimuli were separated by a stimulus-onset asynchrony (SOA) of 1,555 ms.
The ERP from the MMN paradigm was calculated using standard and deviant tones by averaging over trials and baseline-corrected using the time window from 0.1 before until stimulus onset. To calculate MMN, data was extracted from channel FCz (gel EEG cap) and 3Z (dry EEG cap) in the time window of 100 to 150 ms after stimulus onset and amplitude in response to standard stimuli was subtracted from deviant tones. Peak latency and amplitude were determined with the MNE get_peak-function, which determines the maximum amplitude, and its time point in the given time window and channel. In both paradigms, the first 10 trials were used for habituation and excluded from final reporting.
Data Quality
The signal-to-noise ratio (SNR) of the MMN task was compared using a dependent t-test, where as expected, more channels and trials had to be rejected for dry EEG as compared to gel EEG. The SNR was also lower for dry EEG as compared to gel EEG.
Mismatch Negativity Task
Mean amplitude responses were plotted to standard and deviant tones as well as represented in topographical maps for both gel and dry EEG headsets. The results demonstrate that MMN could be detected by both systems, however the mean difference between standard and deviant tones differed between wet and gel EEG, explaining the difference in interaction. While there was no difference in peak amplitude of the MMN task, the amplitude was reached significantly earlier by the dry EEG headset as compared to the gel EEG headset. The topoplot shows that the results for all fronto-central electrodes in both caps were similar to the FCz and 3Z electrodes that the MMN was displayed for. (See Figure 1)
Sensor-Level Resting State Connectivity
The Bland–Altman bias is reported for PLI and MST-Diameter with a 95% confidence interval separately for each frequency band. In theta frequency, the PLI measured with dry EEG was slightly higher, but similar to gel EEG (95% confidence interval of bias includes zero). Overall, PLI was within the limits of agreement for most values except three extreme values for lower frequency band, and the effect sizes show that the differences in PLI range from small (theta) to large (delta), while the differences in MSTDiameter are small or even negligible except for delta frequency. (See Figure 2)
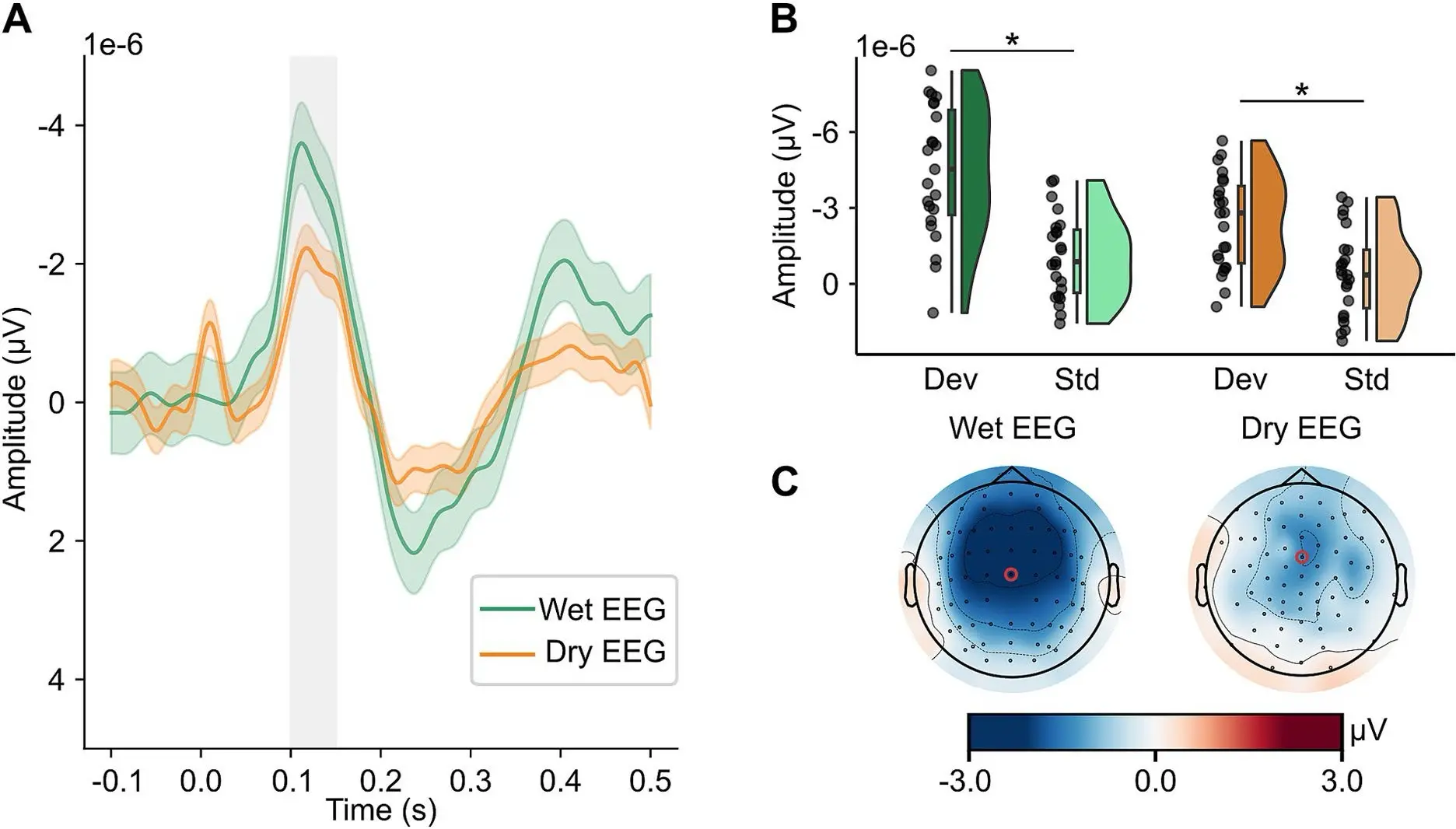
Figure 1: Mismatch Negativity Task. Reproduced from Ehrhardt, Nina M., et al. “Comparison of dry and wet electroencephalography for the assessment of cognitive evoked potentials and sensor-level connectivity.” Frontiers in Neuroscience 18 (2024): 1441799.
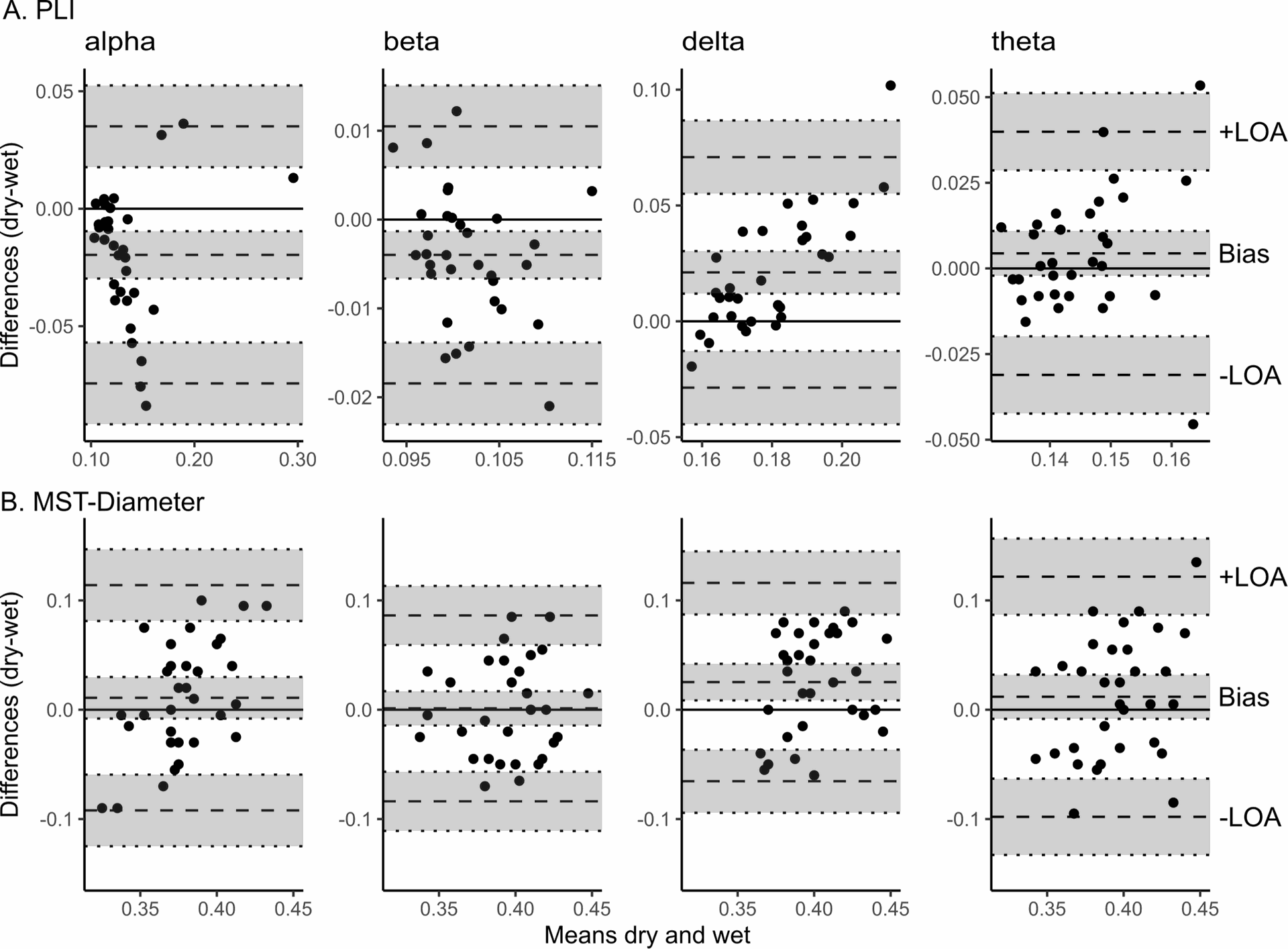
Figure 2: Sensor-Level Resting State Connectivity, Reproduced from Ehrhardt, Nina M., et al. “Comparison of dry and wet electroencephalography for the assessment of cognitive evoked potentials and sensor-level connectivity.” Frontiers in Neuroscience 18 (2024): 1441799.
Amplitude Coupling Is Altered in Delirium of Various Etiologies: Results from a Retrospective Multi-Center Case-Control EEG Study
In this study, a common pathway such as decreased EEG phase coupling is suggested as a comparable clinical measure for delirium. The study explores whether amplitude coupling, or other modes of neural communication get altered in delirium due to different etiologies. All participants including controls were enrolled in center-specific delirium studies under which neurophysiological data was collected from them.
AEC Differs Between Delirious and Non-Delirious Patients
The AECc significantly differed in the delta (padj<0.01) and beta (padj=0.04) band between delirious and non-delirious patients. Median AECc in the delta band was 0.16 (interquartile range (IQR): 0.11–0.21) in delirious patients and 0.12 (IQR: 0.10–0.16) in non-delirious subjects. The amplitude envelope correlation for various frequency bands and delirium types of various etiologies was compared in delirious patients with different etiologies, however, no significant difference in the delta, theta and beta band (all p>0.05) were found. Both studies report significant potential for dry EEG usage clinically and understand the need to reject bad channels due to sudden impedance changes likely caused by low skin-to-electrode contact. However, the appeal for robust and mobile sensor technologies to record in ecologically valid settings still upholds the popularity and convenience of bringing dry EEG methods into clinics.
In Conversation with Dr. Fleischmann
Dry EEG offers several important advantages when applied to poststroke patients or individuals experiencing delirium. In these clinical populations, time is often limited and patient cooperation can be unpredictable. The rapid setup of dry EEG—without the need for skin preparation or conductive gel—allows for quick, non-invasive recordings even in acute settings. This is particularly valuable when working with patients who may be confused, agitated, or cognitively impaired. From a patient perspective, dry EEG is more comfortable and less intimidating, which can increase willingness to participate in repeated assessments. Clinically, this opens new possibilities for longitudinal monitoring and real-time decision support, especially in wards where conventional EEG is logistically difficult. In our experience, the increased ease of use not only improves data collection but also facilitates broader adoption among staff and patients alike. As a result, dry EEG has the potential to integrate neurophysiological diagnostics more naturally into routine care for complex, vulnerable patients.
While dry EEG technology has made impressive strides and proved feasible in clinical populations, there is still room for improvement to support its broader implementation. One important step would be to conduct more large-scale, systematic studies comparing dry and gel EEG across a range of paradigms and patient populations. These should not only assess signal quality but also explore the reliability of dry EEG in detecting clinically relevant features—especially in dynamic or noisy environments. Such comparative data would strengthen confidence in dry EEG’s utility for both clinical and research applications.
Equally important is clear communication about what dry EEG is best suited for. It should not be positioned as a one-to-one replacement for gel EEG in all scenarios, but rather as a powerful tool for largescale data collection—particularly in biomarker research, biobanking, or multi-center studies where ease of use and subject compliance are crucial. Labeling dry EEG explicitly as a high-throughput technology for standardized, population-level data acquisition could help position it more accurately and drive appropriate adoption in both research and clinical contexts.
One of the most appreciated aspects was the quick and straightforward application process, which eliminated the need for time-consuming and often uncomfortable preparations such as abrasive skin cleaning or gel application. Especially in clinical populations, where fatigue, cognitive impairment, or reduced tolerance are common, this streamlined procedure was seen as a major advantage. Patients described the experience as less invasive and more acceptable overall, which also contributed to reduced anxiety and greater willingness to undergo repeated sessions. These subjective impressions were echoed by clinical staff, who noted improved cooperation and reduced dropout rates, particularly among vulnerable individuals such as those with delirium or post-stroke impairments.
The ongoing evolution of dry EEG technology, particularly in terms of sensor fixation and electrode design, is certainly welcome, but in practice I wouldn’t consider this a major limiting factor. When evaluating sensor-level data, especially in the context of spectral power or connectivity analyses, we are typically dealing with widespread electric fields. Small displacements of a few millimeters in electrode position are unlikely to meaningfully impact the results.
For more localized assessments such as source localization, accuracy depends less on the specific electrode model and more on the availability and precision of the montage. As long as the electrode positions are known and well-registered, all modern systems, dry or wet, can support reliable source estimation. Additionally, the use of virtual electrodes allows for interpolation between layouts, making it possible to align data across different systems.
Ultimately, it is important to remember that the electrical field recorded at the scalp is not a direct line from the source to the sensor. It is shaped by multiple biophysical and anatomical factors. Variations in sensor layout play a role, but far more critical for high-resolution modeling is the number of electrodes used and the quality of the reference and head model. So while improvements in electrode design are certainly helpful, they should be seen as part of a broader system, not the decisive factor in data quality or interpretability.
Dry EEG technology holds great promise for a range of clinical populations beyond those already studied. In particular, patients who are intolerant of lengthy or physically demanding procedures such as individuals with long COVID, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), or post-exertional malaise (PEM) could benefit significantly. These groups often experience rapid fatigue, sensory hypersensitivity, or limited physical resilience, making traditional gel-based EEG setups impractical or even distressing.
Moreover, dry EEG is ideally suited for large-scale data collection efforts such as biobanking or AI-driven research into neurological and psychiatric conditions. These approaches require standardized, reproducible acquisition across thousands of samples where short setup times and minimal burden on participants become critical. The ability to efficiently gather high-quality EEG data at scale is a prerequisite for developing robust biomarkers and training reliable predictive models. In this context, dry EEG represents not just a convenience but a strategic enabler for the future of translational neuroscience.
Materials Used
The first study reported in this showcase makes use of 64 channel eego™ systems where data was recorded using a waveguard™ original cap in 10/10 layout as well as a waveguard™ touch cap in equidistant layout for easy comparison. All data was recorded using the eego™ software.
References

Comparison of dry and wet electroencephalography for the assessment of cognitive evoked potentials and sensor-level connectivity
by Ehrhardt, Nina M.
Read More
Amplitude coupling is altered in delirium of various etiologies: Results from a retrospective multi-center case-control EEG study
by Fleischmann, Robert.
Read More